A MedMMAP Critical Process Assessment is based on MedAccred’s industry-approved criteria and includes a review of the procedures, work instructions, training records and other documentation that evidences the competency of the company to meet customer requirements.
A Critical Process Assessment also involves observing the critical processing through job audits to ensure that documented requirements are properly flowed down to and implemented on the shop floor.
A job audit is a step-by-step review of the critical process for producing an item and evaluating how the customer requirements are met by using the MedAccred audit criteria.

Medtronic
Baxter
Philips
Johnson & Johnson
BD
Stryker
Bausch Health
Boston Scientific
Roche Diagnostics
Edwards Lifesciences
*audit criteria under development
Results from the Critical Process Assessment are used by MedMMAP and the company to develop and execute a plan to close all non-conformances and root cause corrective actions.
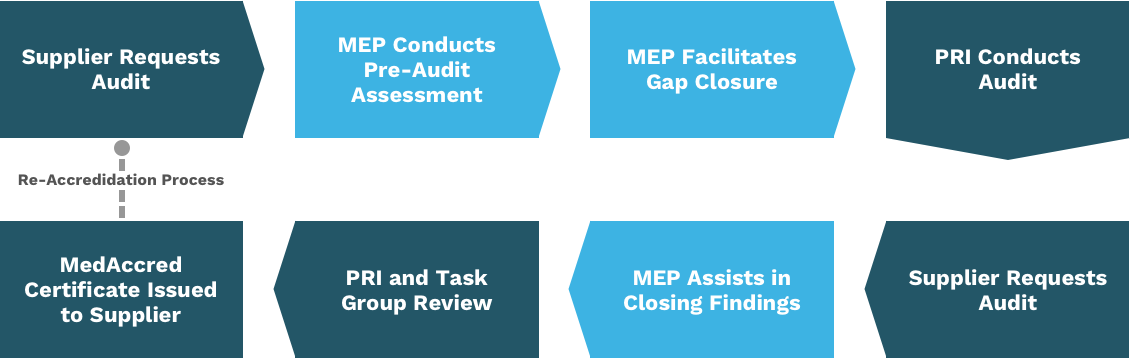
The MedMMAP Critical Process Assessment meets the MedAccred requirement that a supplier perform an internal pre-audit prior to the accreditation audit.


© 2024 MedMMAP. All Rights Reserved.